Cellular senescence is a state of stable cell cycle arrest under which cells remain metabolically active, but no longer divide and do not respond to growth-promoting stimuli. Senescence is triggered by a variety of cellular stressors. These include environmental factors like ionizing radiation or exposure to chemotherapeutic drugs, oxidative stress, DNA damage, mitochondrial dysfunction, and oncogene activation. This process provides a defense mechanism to maintain tissue homeostasis through the sequestration of damaged cells. Senescent cells influence a number of physiological and pathological processes from cancer to diabetes and aging. Accordingly, understanding why senescence contributes to these conditions may lead to the development of pro- and anti-senescence therapies to treat a range of diseases.
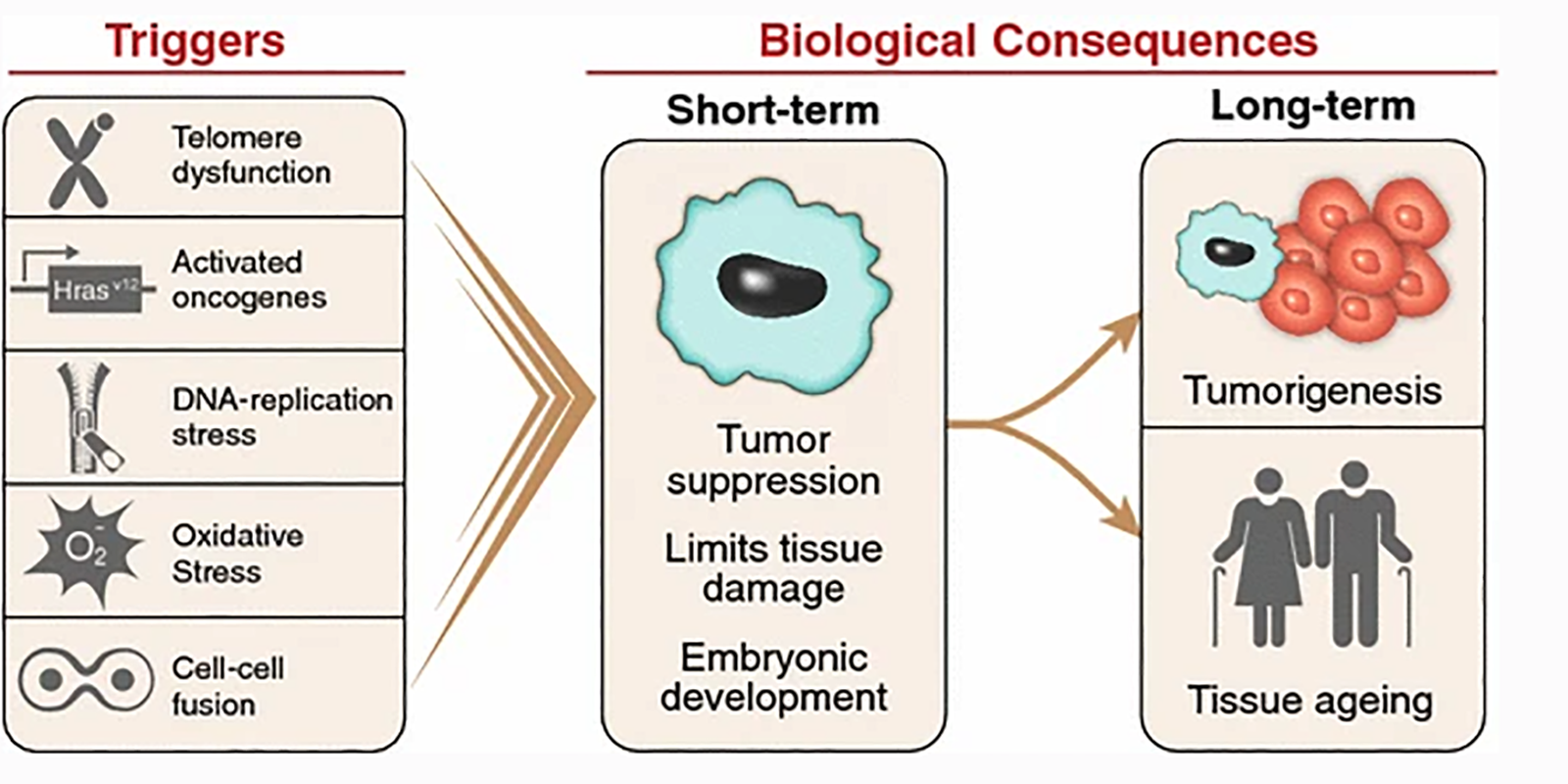
DNA damage or other senescence-inducing stimuli induce cell cycle arrest through the activation of the p53/p21CIP1 or p16INK4A/phospho-Rb tumor suppressor pathways. These cascades induce senescence by inhibiting kinases associated with cell cycle progression and blocking the transcription of proliferation promoting genes. In addition to permanent withdrawal from the cell cycle, senescent cells exhibit several characteristic features including morphological changes, activation of the senescence-associated secretory phenotype (SASP), and evidence of DNA damage.
Morphologically, cells that have undergone senescence have an enlarged and flattened shape and disrupted nuclear envelope integrity due to a decrease in the expression of Lamin B1. The formation of senescence-associated heterochromatin foci (SAHF) is a common form of chromatin reorganization observed in cells undergoing oncogene-induced senescence. In addition, b-galactosidase accumulates in senescent cells due to altered lysosomal activity.
Senescent cells influence the surrounding tissue microenvironment through dramatic changes in the proteins they secrete, a phenomenon known as the senescence-associated secretory phenotype (SASP). The SASP encompasses the upregulation and release of growth factors, cytokines, and proteases that can exert positive or detrimental effects depending on the cellular context. For example, SASP can initiate tissue repair and the removal of damaged cells by recruiting and activating immune cells. Conversely, SASP can promote tumor growth and progression by stimulating angiogenesis and ECM remodeling. Specific examples of factors released by the SASP include interleukin-6, interleukin-1b, matrix metalloproteinase-3, and chemokine (C-X-C motif) ligand 10, among others.
Senescent cells accumulate with age and contribute to the normal aging process as well as age-related disorders. Research in animal models has shown that in vivo administration of compounds called senolytics, which selectively eliminate senescent cells, can reduce inflammation, enhance immune system function, and thereby slow the progression of aging – leading to increased health and lifespan. Conversely, factors that activate senescence have shown some efficacy in treating certain types of cancer by suppressing tumor cell growth and proliferation. However, the off-target accumulation of senescent cells following chemotherapy may contribute to unwanted side effects, including fatigue and a propensity for cancer relapse due to the release of SASP components. Therefore, a complete understanding of the mechanisms of senescence is required to exploit the balance between its beneficial and harmful effects to effectively treat pathological states and potential slow the progression of aging.
For more information on cellular senescence, its signaling pathways, and assays to study senescence in your research, please visit the CST Senescence Signaling pathway.


